NPDB Insights
Is It Reportable?

A physician applying for renewal of his hospital clinical privileges falsified his application by omitting information about an ongoing licensure investigation. The hospital took a professional review action to deny his renewal application, which the medical executive committee considered to be related to the practitioner’s professional conduct, even though there was no actual patient harm. Should this be reported to the NPDB?
A clinical privileges action must be reported to the NPDB if it is the result of a professional review action that relates to professional competence or conduct that adversely affects, or could adversely affect, the health or welfare of a patient and lasts for a period longer than 30 days. Whether an action affects or could affect patient health or welfare is generally a determination that must be made by the entity taking the action. If, in the opinion of the medical executive committee, the practitioner’s falsification of his application could adversely affect the health or welfare of a patient, and the action is the result of a professional review, the action must be reported to the NPDB.
Reporting Flowcharts Simplified as Infographics
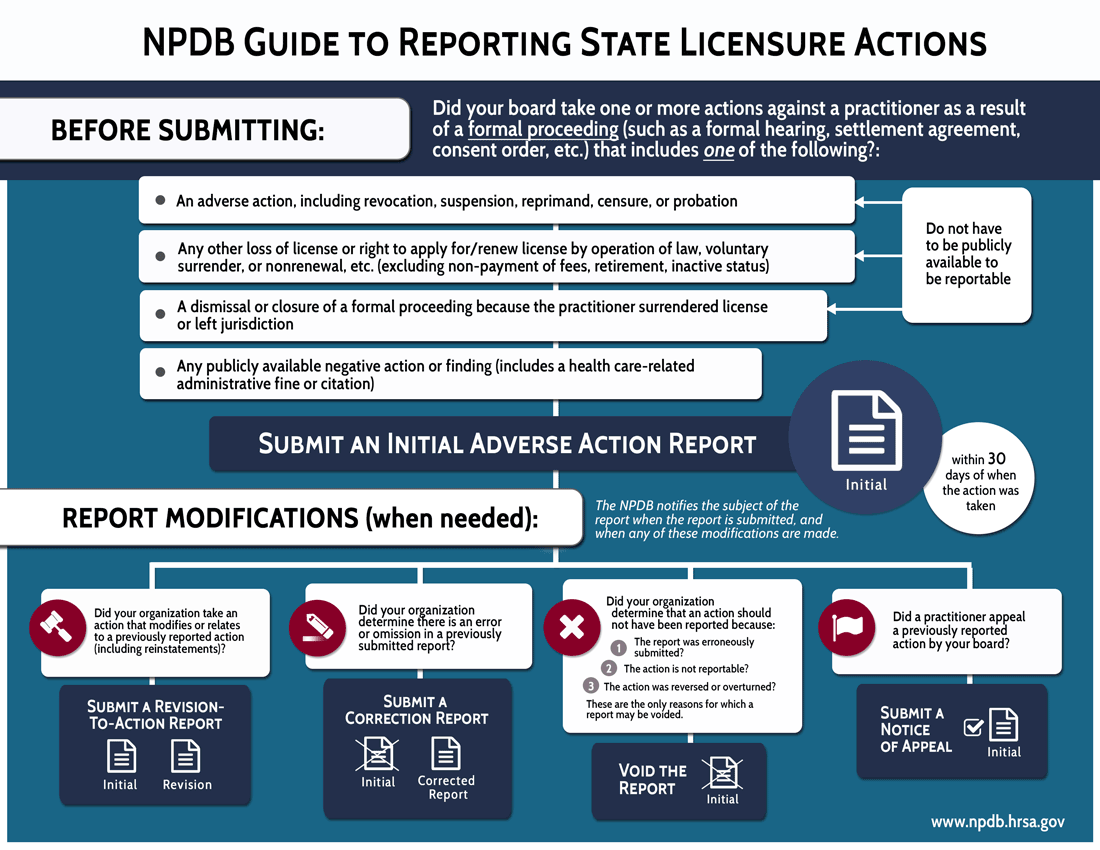
The NPDB has redesigned two of the most popular reporting flowcharts: state licensing board actions, and clinical privileges actions. Both infographics focus on the conditions for reportability (in the top half), and the reasons for modifying a report such as correcting a report, voiding a report, or submitting a revision-to-action report (in the bottom half).
The state licensure actions infographic explains the conditions for reporting state licensure actions:
- It must be taken through a formal proceeding (e.g., formal hearing, settlement agreement, consent order, etc.), AND
- Include one of the four conditions listed in the infographic.
The clinical privileges infographic highlights the key conditions a professional review action must fulfill in order to be reportable as an initial adverse action report, namely:
- It must adversely affect clinical privileges (including privileges, medical staff or panel membership, network participation, and other circumstances) for a period of more than 30 days, AND
- Be based on the practitioner’s professional competence or professional conduct that adversely affects, or could adversely affect, the health or welfare of a patient.
The NPDB will add more reporting and querying guides to our Infographics page, which also houses "What is the NPDB?", "A Practitioner's Guide to the NPDB", "3 Reasons to Activate Continuous Query" and the August and November 2016 Compliance Maps.
The Importance of Proper Coding

The NPDB recently released new guidance in the Policy Corner, "Basis for Action Codes: Why Accurate Coding is Critically Important", which provides a brief explanation of why reporters must use the codes list, details the role of reporters in NPDB operations, and more.
A codes list is provided for all reporting entities to use in selecting the type of action taken and the basis for the action. When submitting a report, it is important that the most accurate codes are selected, and multiple codes can be selected if there are multiple reasons for the actions taken. There are many possible choices, and there is also an "Other" code. "Other" should only be used if there are no codes that match the actual basis for action.
Accurate coding when querying or reporting is critical when describing, for example, the type of practitioner, the reportable action, or the purpose of a query. In turn, accurate reporting and querying results in better information for protecting the public and in fulfilling the mission of the NPDB.
Our goal is to make NPDB policies easier to understand and to provide clarification to the current guidance for users. If you have any questions about NPDB's policy and expectations on the use of reporting codes, you can email NPDBCompliance@hrsa.gov.
The latest updates and resources are available at http://www.npdb.hrsa.gov.
Previous editions of NPDB Insights are available in our archive.
 An official website of the United States government.
An official website of the United States government.